通過韓國有機認證 Receive Korean Organic Certification
高純度果寡糖產品通過韓國有機認證,擁有四重有機認證(美國、歐盟、中國及韓國)
High purity FOS powder receives Korean Organic certification, having four organic certifications ( USDA, EU, China and Korea)
推出「超低水活性系列」Launch a “Super Low-Aw series”
為提供更適合粉劑保健品產品原料,推出超低水活性低的功能性寡糖原料-GOS/FOS
In order to provide more suitable material for healthy food powder, we launch a series of prebiotics GOS/FOS products with super low water activity
推出「飼益元」品牌 “Feedligos” a brand for feed-grade products
為滿足益生元於動物保健的市場需求提高,推出「飼益元」品牌系列產品
To satisfy the need of the animal health market, we a launch “Feedligos” to focus on animal health.
取得飼料添加劑生產許可證 Receive Feed Additives Production License
粵飼添(2020)H21002 / 粵飼添(2020)T21002
Guangdong Feed Additives (2020) H21002/ T21002
GMP新廠計畫啟動 GMP Plant under Construction
選址於河口醫藥園區,採用國際GMP標準設計,預計2021年正式啟用
Located in the He-Kou health pharmaceutical industry park, it is to be expected to be completed in 2021.
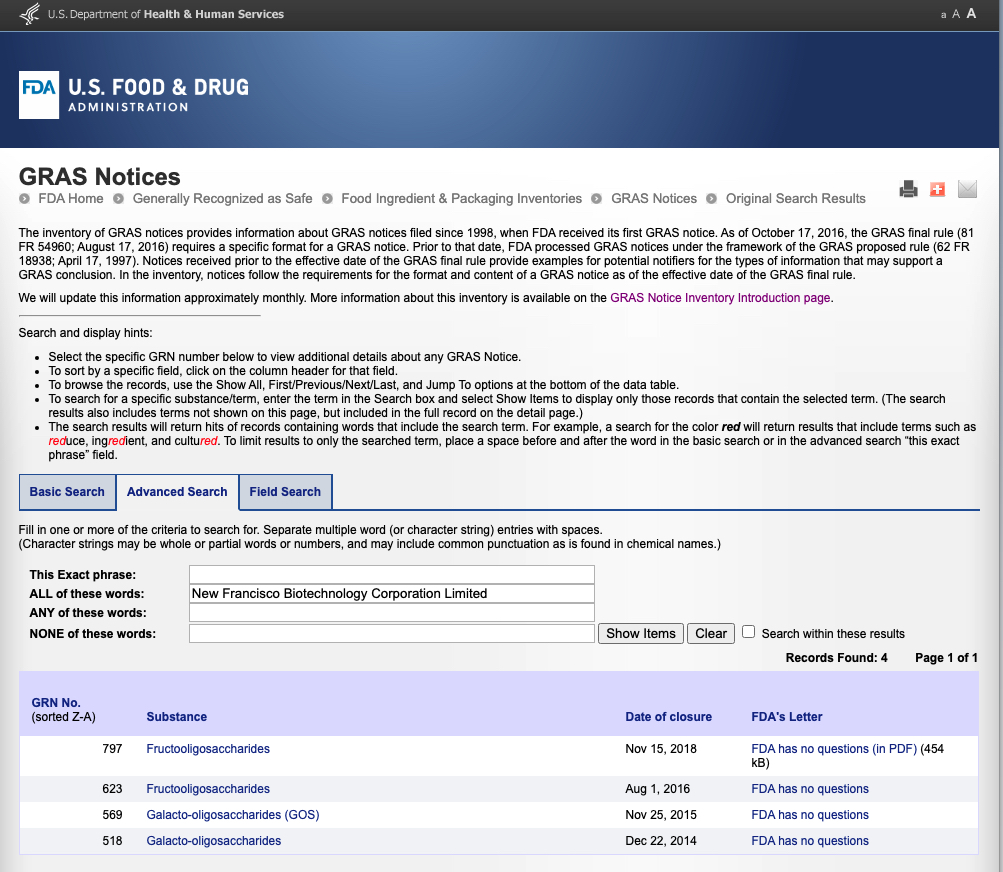
取得美國GRAS No. 797 Receive FDA GRAS no. 797
果寡糖產品針對嬰幼兒食品應用,取得美國FDA GRAS認證號碼797
FOS products have received FDA GRAS approval for use in Infant food.
中國新三板上市 Public Listing on China OTC Market
於北京證交所掛牌上市,股票代碼(871395)
Officially listing at Beijing stock exchange (871395)
獲得中國有機認證 Obtained China Organic Certification
半乳寡糖及果寡糖產品通過中國有機認證,為全球唯一三重有機認證的寡糖生產商
GOS and FOS products have obtained China organic certification, and are the only oligosaccharide manufacturer with three organic certifications (EU, NOP and China organic certificate).
通過高新技術企業認定 Received High-Tech Enterprise Certificates
同步成立中國首省級“低聚半乳糖工程技術研究中心”
Simultaneously established the first provincial-level “Galacto-oligosaccharides technology and process research center” in China
通過美國及歐盟有機認證 Obtained the EU and NOP organic certificates
半乳寡糖、果寡糖、異麥芽寡糖系列產品及澱粉糖產品皆通過美國USDA及歐盟有機認證,為全球首家獲得雙有機認證的寡糖生產商
GOS, FOS, IMO and starch sugar products have obtained the USDA and EU organic certifications. The first prebiotic manufacturer in the world to receive both NOP and EU Organic Certificates
世界乳品創新獎認可 Winner in “Best Dairy Ingredient” of World Dairy Innovation Award 2016
以100%GOS產品獲得全球最佳乳品配料首獎,並入圍最佳純化技術獎
King-Prebiotics® 100% Galacto-oligosaccharide has been announced the winner in “Best Dairy Ingredient” of World Dairy Innovation Award 2016 and was nominated for the best purification technology award
取得美國FDA GRAS安全認證 Receive US FDA GRAS Approvals
半乳寡糖GOS產品取得美國GRAS號碼No.518 (一般食品應用) & No. 569 (嬰幼食品應用)
果寡糖FOS產品取得美國GRAS號碼No.623 (一般食品應用)
*GRAS (Generally Recognized As Safe)
GOS products have received FDA GRAS approval for use in general food & Infant food GRN.518 & 569
FOS products have received FDA GRAS approval for use in general food GRN. 623
主導半乳寡糖的GB制定 Participating the Establishment of National Standard (GB) of GOS
參與中國衛生部召集制定中國低聚半乳糖的國家標準(GB)
Participated in the National standard (GB) of Food safety for Food additive Galacto-oligosaccharides convened by the Ministry of Health of China
開發100%純度半乳寡糖 Developed of 100% pure Galactooligosaccharides
領先全球,研發出100%純半乳寡糖產品(GOS-1000-P),適用於血糖調節及乳糖不耐等相關應用,率先將益生元的應用跨入保健及醫藥領域
Leading the world, launching King-Prebiotics® GOS-1000-P, which is a 100% pure GOS specially designed for diabetes and lactose-intolerance patients, developing new applications of prebiotics into nutraceutical and pharmaceutical industries
譽為「中國低聚糖行業的領航者」 Honored as “The pioneer In Chinese Oligosaccharides Industry”
作為首家導入半乳寡糖的企業,並擁有全球唯一加工食品添加後半乳寡糖含量檢測技術,榮幸受中央電視台CCTV-4獨家報導,被譽為「中國低聚糖行業的領航者」
As the first company to import galacto-oligosaccharides and possesses the world’s only technology for detecting the content of galacto-oligosaccharides in Food additives, it is honored to be interviewed exclusively by CCTV-4 and honored as “The pioneer In Chinese Oligosaccharides Industry”
中國首家獲得QS認證 The first company in China to obtain QS certification
主導半乳寡糖生產許可審核及細則制定,是中國首家獲得QS認證的半乳寡糖合格製造廠
Played a key role in the discussion of establishing the regulation of Galacto-oligosaccharide production license and audit regulation, is the first qualified galacto-oligosaccharide manufacturer in China to obtain QS certification
導入功能性寡糖 Introduced functional oligosaccharides
陸續導入異麥芽寡糖、果寡糖、半乳寡糖和高果糖漿
Introduction of Isomaltooligosaccharides (IMO), Fructooligosaccharides (FOS), and Galactooligosaccharides (GOS)